San Francisco & Paris. March 5, 2025 — hinlab, a medical technology company specializing in cardiology, announces that its first product, hinscope, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This clearance confirms that hinscope complies with the highest standards of safety, quality, and performance, marking a significant milestone for hinlab as it enters the U.S. market, one of the most demanding and dynamic sectors in the healthcare industry.
The FDA's 510(k) clearance for hinscope comes at a pivotal time as demand for connected health solutions continues to rise. hinscope offers a reliable and innovative approach to addressing the challenges of cardiovascular disease, with a primary objective of promoting early diagnosis at the point of care.
hinlab's team, consisting of data scientists, engineers, and medical professionals, is committed to tackling the complexities of cardiovascular care. Through a combination of proprietary hardware devices equipped with advanced sensors and deep learning/artificial intelligence algorithms, hinlab is developing one of the most sophisticated AI-enhanced cardiology platforms available. This solution aims to improve clinicians' ability to accurately identify, triage, and diagnose patients efficiently, all while addressing the increasing need for scalable healthcare solutions.
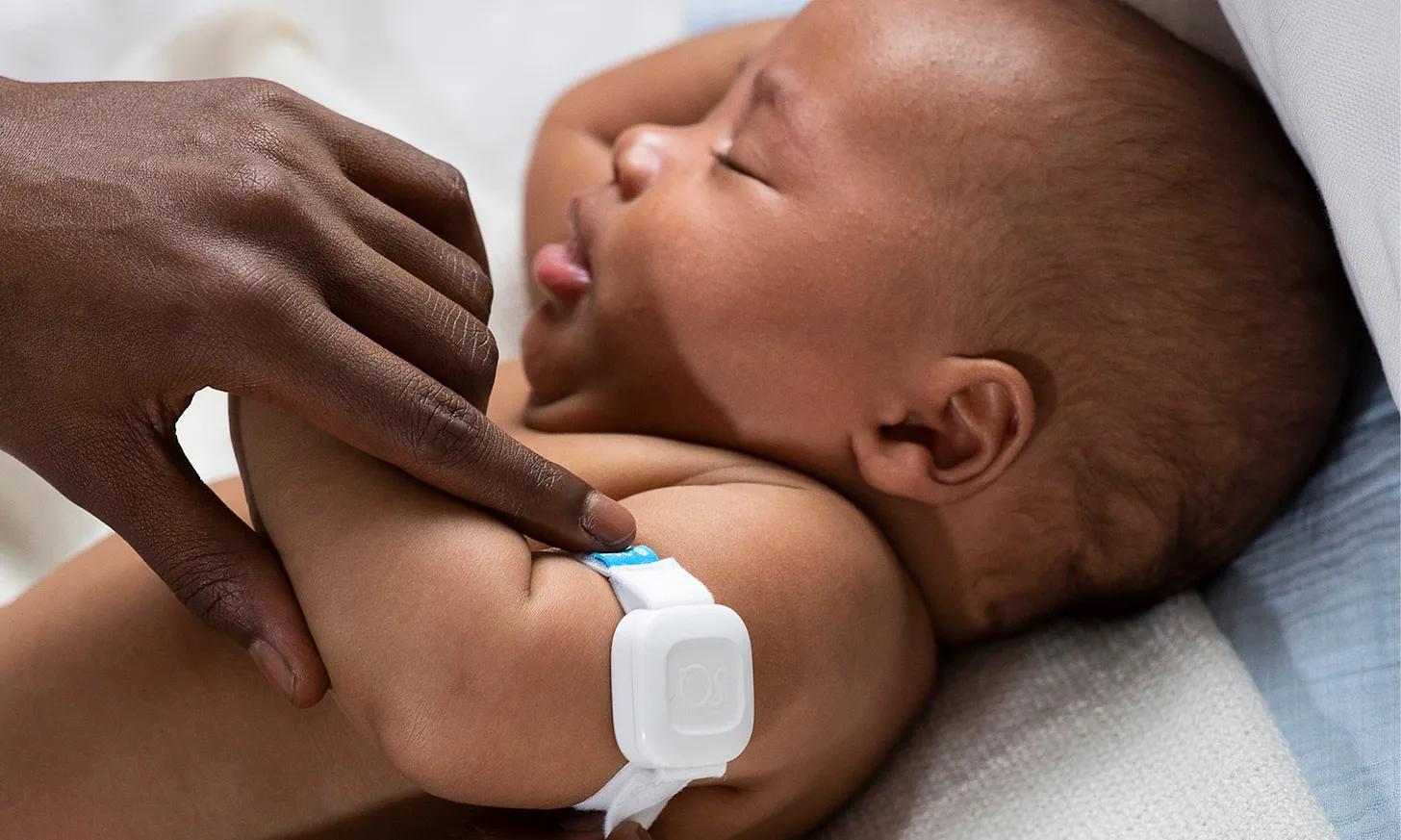
As part of this platform, hinscope is a first-of-its-kind connected device for monitoring key patient vital signs. After rigorous clinical trials, electrical safety testing, cybersecurity evaluations, electromagnetic compatibility testing, and biocompatibility assessments, hinscope has successfully met the standards required for FDA 510(k) clearance.
This achievement is a testament to hinlab’s dedication to excellence and innovation, driven by a talented and agile team with deep expertise in regulatory compliance, algorithms, electronics, software, and clinical research. The approval underscores the company’s ability to meet stringent regulatory standards and lays the groundwork for future success.
The 510(k) clearance grants hinlab access to the U.S. market, enabling the company to collaborate with key healthcare stakeholders to deploy this technology at scale. This strategic milestone also provides an opportunity to attract investors and demonstrates the company's ability to navigate complex regulatory challenges.
About hinlab
hinlab Inc. is a global medical technology company based in San Francisco, California and Paris, France. Our mission is to make advanced cardiac care more accessible, improving diagnostics and treatment for cardiovascular diseases.